Synthetic surgical glove
Biogel PI UltraTouch S
Biogel® PI UltraTouch® S is a surgical glove made from synthetic polyisoprene not made with chemical accelerators known to cause contact dermatitis

Biogel® PI UltraTouch® S is a surgical glove made from synthetic polyisoprene not made with chemical accelerators known to cause contact dermatitis, such as Thiazoles, Thiurams, Carbamates, Thioureas and Diphenylguanidine
- Manufactured without chemical accelerators known to cause contact dermatitis
* - Reduced risk of a hole with an industry-leading AQL** of 0.65, determined post packaging
- Every glove (100%) is air-inflation tested for holes typically not detected in a visual inspection
- Low endotoxin level (<20 EU/pair), which may reduce the risk of post-operative complications
*Thiazoles, Thiurams, Carbamates, Thioureas and Diphenylguanidine
**AQL = Acceptable Quality Level refers to the maximum number of defective products that could be considered acceptable during the random sampling of an inspection, in this case freedom from holes in gloves.
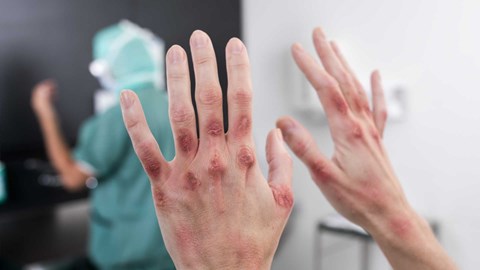
Contact dermatitis is an inflammation of the skin resulting from direct contact of a substance with the surface of the skin
Related products
Surgical teams around the world value Biogel® gloves for their comfortable fit and precise feeling
'References'













