IIS Admission Process
The IIS admission process consists of two major steps: first you will need to submit a concept proposal, which should be followed by the submission of a full proposal in case of approval. Read below about the specific steps.
STEP I: CONCEPT PROPOSAL
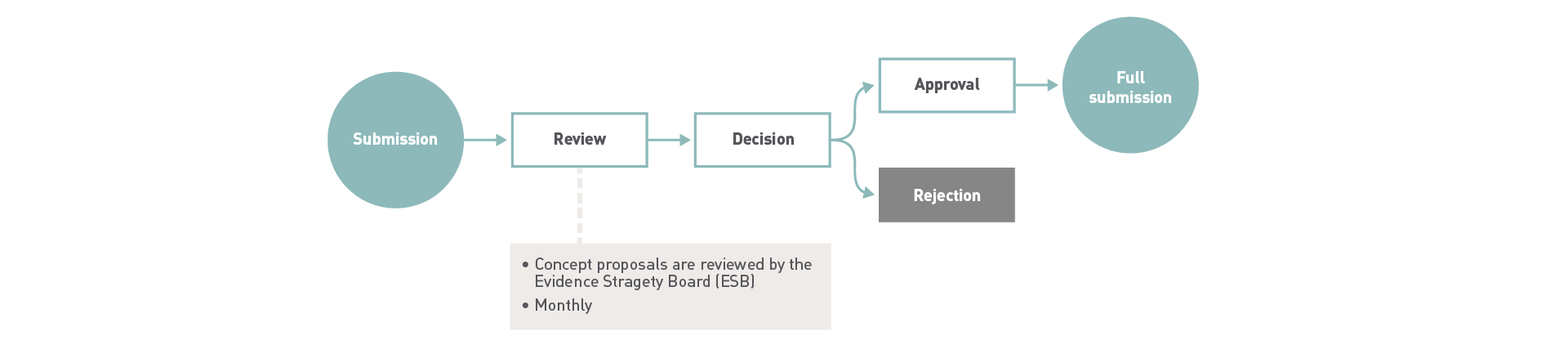
- We have continuous admissions for concept proposals.
- All proposals are reviewed by our Evidence Strategy Board (ESB).
- Applicants will get an answer approximately 8 weeks if the proposal converges with our research focus.
STEP II: FULL PROPOSAL
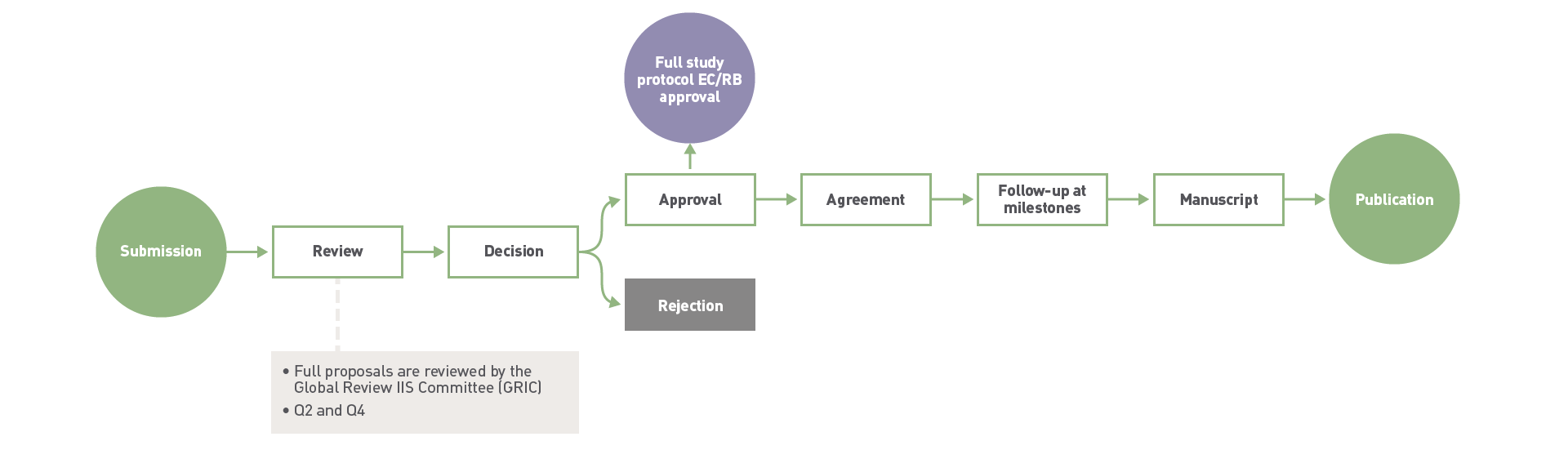
- We have continuous admissions for full proposals.
- All full proposals are reviewed by our Global Review IIS Committee (GRIC) during 2 and 4 quarter.
- Applicants will get an answer by the end of the GRIC’s review period.
How to submit your proposal
If you have a proposal for a research project please feel free to contact us at investigatorinitiatedstudies@molnlycke.com submitting all the necessary documentation (see below).
We support high quality projects that always put patient safety first. For this reason, approvals (or waivers) from an applicable Research Ethics Committee (REC) / Institutional Review Board (IRB) are part of our process when we review requests for IIS support.
The study proposal should include the information detailed in the Study proposal form that you can download below. Our research grant is provided as financial support of all or a portion of study‐related costs and/or investigating product(s). Since this type of project is sponsored and owned by the investigator, the financial support excludes overhead.
Your proposal will of course be treated with confidentiality.
Kindly submit your proposal in English in one of the following formats: PDF, jpeg or png.
For more information on how we process your data see our privacy notice.
Download the form to submit concept or full proposal
Download the budget template (for full proposal)
What happens after submission
Supporting a research proposal is a big step for us and for you. So we want you to know how we make decisions and what to expect after you submit your proposal.
Step I: concept proposal
1. We’ll confirm when we’ve received your proposal and if we need more information, we’ll ask you for it.
2. Once we’ve received the information we need, we’ll review your proposal, looking at: safety of the participating subjects, alignment with Mölnlycke clinical and health economic evidence strategy, study design, statistics, probability that the study will lead to a meaningful publication in a peer review journal, your qualifications and scientific merits and your budget request.
3. You may be asked additional questions before we can make a decision about supporting your proposal.
4. Within 8 weeks, you’ll receive a written response with a rationale for our decision. We may decide to reject or approve your proposal. An approval means that your proposal has been selected to proceed to step two, which involves submission of a full IIS application.
Step II: full proposal
1. We’ll confirm when we’ve received your proposal. And if we need more information, we’ll ask you for it.
2. Once we’ve received the information we need, we’ll review your proposal, looking at: safety of the participating subjects, alignment with Mölnlycke clinical and health economic evidence strategy, study design, statistics, possibility that the study will lead to a meaningful publication in a peer review journal, your qualifications and scientific merits and if your budget request is within fair market value.
3. You may be asked additional questions before we can make a decision about supporting your proposal.
4. At the end of the review period, you’ll receive a written response with a rationale for our decision. We may decide to reject your proposal, offer a conditional approval or give you final approval.
5. Once the approval is in place, we’ll prepare our IIS Financial Support Agreement, describing the responsibilities of everyone involved.
6. During your research, follow-ups at start, in the middle, and end of the study that are linked to the milestones (often correlated with the number of enrolled subjects) will be requested from you.
7.The final and important step is the reporting and publication. We will then ask you to share your results, abstract / manuscript, including the final version for submission. If you have any questions, please feel free to contact by sending an e-mail to investigatorinitiatedstudies@molnlycke.com.
All IIS investigators are invited to apply for Mölnlycke’s IIS dissemination grant related to the IIS study in question including:
- medical writing and statistical analysis fee,
- travel fee for presenting IIS study at conferences,
- publication fee.
Download the form to apply for IIS dissemination grant
We only support study proposals after receiving a copy of the Ethics Committee/IRB favorable opinion/approval for the study.
For further information regarding regulatory requirements that Mölnlycke adheres to, please see our IIS governance references.





