Synthetic surgical glove
Biogel PI UltraTouch S

Biogel® PI UltraTouch® S is a synthetic polyisoprene surgical glove not made with chemical accelerators known to cause contact dermatitis, such as Thiazoles, Thiurams, Carbamates, Thioureas and Diphenylguanidine
This glove is also tested for use with chemotherapy agents per ASTM D6978.

- Cleared to reduce the potential of sensitizing users to chemical additives
- AQL of 0.65 (freedom from holes), determined post packaging
- Every glove (100%) is air-inflation tested for holes typically not detected in a visual inspection
- Low endotoxin level (<20 EU/pair), which may reduce the risk of post-operative complications
For information tailored to your market, please click on the the button below.
Attention: Products shown on the Mölnlycke web sites(s) may not be available in all markets and product indication claim(s) may vary between markets.
Our commitment to precision
At Mölnlycke, everything we do is about supporting the performance of our customers. This means supplying them with the most reliable – and most precise – equipment possible. This commitment begins with the design and engineering of Biogel® gloves, and flows right through to the OR.
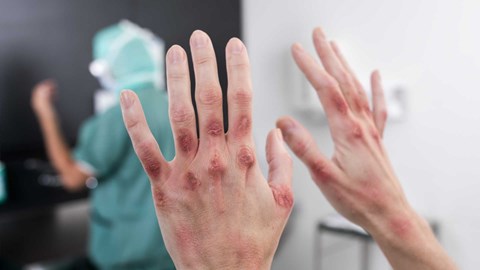
Contact dermatitis is an inflammation of the skin resulting from direct contact of a substance with the surface of the skin
Related products
Surgical teams around the world value Biogel® gloves for their comfortable fit and precise feeling
'References'
















