Mölnlycke launches new skin-friendly Biogel® surgical gloves to reduce the risk of allergic contact dermatitis for surgeons and nurses
Gothenburg, Sweden. 15 October, 2020 – Mölnlycke is celebrating World Handwashing Day with the global launch of the innovative Biogel® PI UltraTouch® S surgical glove, which addresses the problem of allergic contact dermatitis among surgical teams
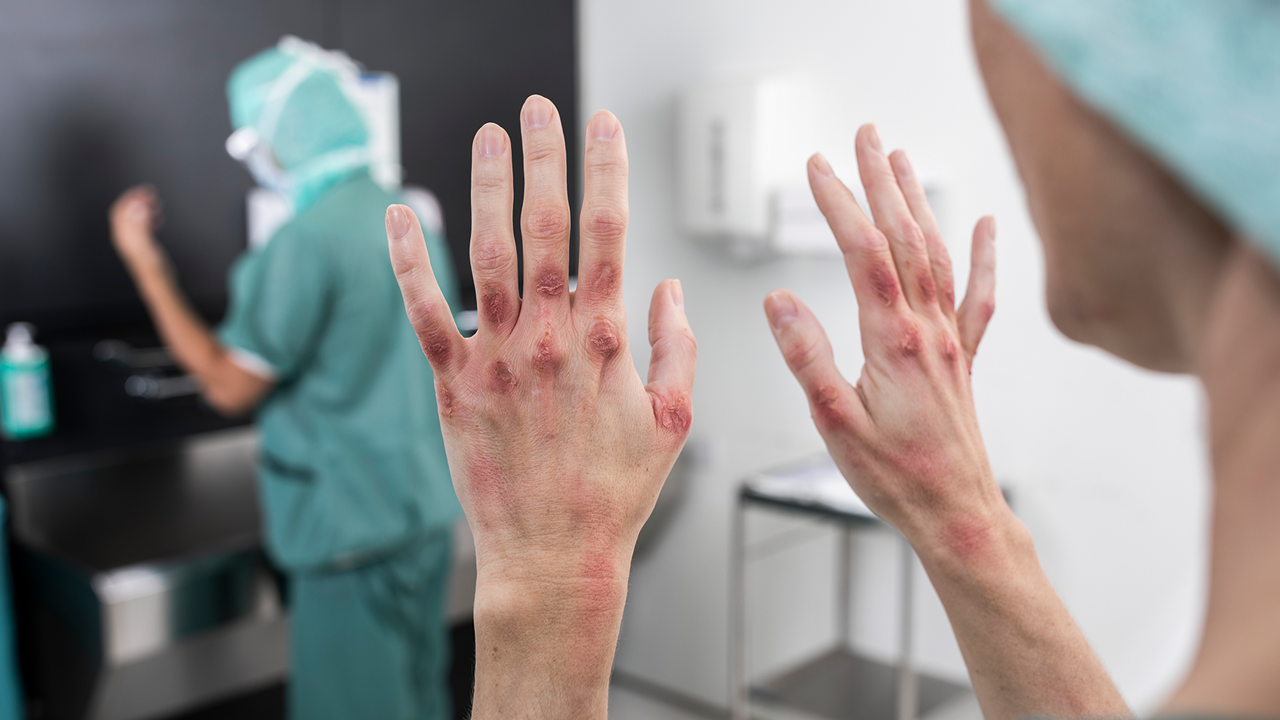
The latest addition to the industry-leading Biogel product line, Biogel PI UltraTouch S surgical gloves have been designed with a new skin-friendly formula that is clinically proven to minimise the risk of allergic contact dermatitis in the operating room.* It is the first surgical glove to be cleared by the FDA to reduce the potential for sensitising users to chemical additives.
Precision without irritation
Offering the same precision, tactile sensitivity and best-in-class perforation detection
With the PI UltraTouch® S, allergic reactions caused by surgical gloves no longer need to be a barrier to performance, allowing healthcare professionals to focus on easing the backlog of elective care procedures caused by the coronavirus crisis, free from unnecessary distractions and harm.
The scale of contact dermatitis among surgeons and nurses
This year, a Mölnlycke-commissioned survey conducted by SERMO, the global network of healthcare professionals, revealed a shocking level of irritant and allergic contact dermatitis among surgeons and nurses working in the US healthcare system
- 31% of surgeons and nurses surveyed had suffered from a skin reaction in the operating room
- 60% of those had experienced a skin reaction in the last year
- 28% of surgeons had been forced to change gloves during a procedure due to a skin reaction
- 22% had been distracted from work due to a skin reaction from surgical gloves
- 49% had seen a colleague distracted due to a skin reaction from surgical gloves
The cost of allergens
Instances of Type IV allergic contact dermatitis place surgeons and nurses at considerable harm and cause severe burdens on hospitals and health systems with lost productivity and occupational treatment costs. The cost of treating a single clinician with a glove-related allergy is estimated to be between USD 1,955 and USD 11,184 per year.
Providing surgeons and their teams with dermatologically appropriate personal protective equipment (PPE) is the vital first step to ensuring a safe, effective and efficient operating room.
Commenting on the launch of Biogel PI UltraTouch S surgical gloves, John Timmons, International Medical Director of Mölnlycke, said:
'In the O.R., precision can be the difference between successful and unsuccessful surgical outcomes. We have engineered these new gloves to not only protect clinicians’ hands, but also to support their precision. It seems fitting to recognise World Handwashing Day with a new way to improve working conditions for surgical team members who suffer from the pain and discomfort of allergic contact dermatitis.'
'Contact dermatitis has long been a distraction to surgeons and nurses in the operating room. With Biogel PI UltraTouch S, these medical professionals now have a high-performing glove that allows them to do the very best job, free from worry about experiencing skin reactions from chemical accelerators. Surgical team members who suffer from allergic contact dermatitis can now enjoy the same precision, fit and safety of other Biogel surgical gloves.'
Biogel PI UltraTouch S surgical gloves are available now to hospitals across the United States. To request a free sample, please visit: www.molnlycke.com/biogel
Biogel PI UltraTouch S has received FDA 510k clearance for reduced potential for sensitising users to chemical additives, the first sterile surgical glove to be awarded this claim. Modified Draize-95 patch testing was completed on 200 participants.
*Manufactured without the chemical accelerators known to cause irritant or Type IV allergic contact dermatitis including Dithiocarbamate (DTC), Diphenyl thiourea (DPTU), Diphenylguanidine (DPG), Zinc mercaptobenzothiazole (ZMBT), Thiurams
For media inquiries, contact:
Michael Latham, Lexington Communications
Michael.latham@lexcomm.co.uk
Tel: +44 (0)20 7025 2354
Jenny Johansson, Mölnlycke
Jenny.johansson@molnlycke.com
Tel: +46 (0)31 722 34 23
About Mölnlycke
Mölnlycke is a world-leading medical solutions company. Our purpose is to advance performance in healthcare across the world. That is why we aspire to equip everybody in healthcare with solutions to achieve the best outcomes. We develop and bring to market innovative wound care and surgical solutions along the entire continuum of care – from prevention to post-acute settings. Our solutions provide value, supported by clinical and health economic evidence.
Mölnlycke employs around 8,000 people. The company headquarters are in Gothenburg, Sweden and we operate in more than 100 countries worldwide. Since 2007, the company is part of Investor AB, an engaged owner of high-quality, global companies which was founded by the Wallenberg family in 1916. For more information, please visit www.molnlycke.com.





